Diamonds
The Chemistry of Diamonds
Carbon
Chemistry and Diamond Crystal Structure
by Anne
Marie Helmenstine, Ph.D.
 The word
'diamond' derives from Greek adamao, meaning 'I tame'
or 'I subdue' or the related word adamas, which means
'hardest steel' or 'hardest substance'.
The word
'diamond' derives from Greek adamao, meaning 'I tame'
or 'I subdue' or the related word adamas, which means
'hardest steel' or 'hardest substance'.
Everyone knows
diamonds are hard and beautiful, but did you know a diamond could be the oldest
material you might own?
This
discrepancy is because the volcanic magma that solidifies into rock where
diamonds are found did not create them, but only transported the diamonds from
the Earth's mantle to the surface.
Diamonds
also may be formed under the high pressures and temperatures at the site of
meteorite impacts.
 The
diamonds formed during an impact may be relatively 'young', but some meteorites
contain star dust, debris from the death of a star, which may include diamond
crystals.
The
diamonds formed during an impact may be relatively 'young', but some meteorites
contain star dust, debris from the death of a star, which may include diamond
crystals.
One such
meteorite is known to contain tiny diamonds over 5 billion years old. These
diamonds are older than our solar system!
START
WITH CARBON
Understanding
the chemistry of a diamond requires a basic knowledge of the element carbon.
A neutral carbon
atom has 6 protons and 6 neutrons
in its nucleus, balanced by 6 electrons.
The electron
shell configuration of carbon is 1s22s22p2.
Carbon has a valence of 4, since
4 electrons can be accepted to fill the 2p orbital.
 Diamond is
made up of repeating units of carbon atoms joined to four other carbon atoms
via the strongest chemical linkage, covalent bonds.
Diamond is
made up of repeating units of carbon atoms joined to four other carbon atoms
via the strongest chemical linkage, covalent bonds.
Each
carbon atom is in a rigid tetrahedral network where it is equidistant from its
neighboring carbon atoms.
The
structural unit of diamond consists of 8 atoms, fundamentally arranged in a
cube.
This
network is very stable and rigid, which is why diamonds are so very hard and
have a high melting point.
Virtually all
carbon on Earth comes from the stars.
Studying the
isotopic ratio of the carbon in a diamond makes it possible to trace the
history of the carbon.
For example, at
the earth's surface, the ratio of isotopes carbon-12 and carbon-13 is slightly
different from that of star dust.
Also, certain
biological processes actively sort carbon isotopes according to mass, so the
isotopic ratio of carbon that has been in living things is different from that
of the Earth or the stars.
Thus it is known
that the carbon for most natural diamonds comes most recently from the mantle,
but the carbon for a few diamonds is recycled carbon of microorganisms, formed
into diamonds by the earth's crust via plate tectonics.
Some minute
diamonds that are generated by meteorites are from carbon available at the site
of impact; some diamond crystals within meteorites are still fresh from the
stars.
CRYSTAL
STRUCTURE
The
crystal structure of a diamond is a face –centered cubic or FCC lattice.
Each carbon atom
joins four other carbon atoms in regular tetrahedrons (triangular prisms).
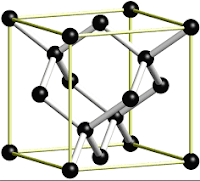 Based on the
cubic form and its highly symmetrical arrangement of atoms, diamond crystals
can develop into several different shapes, known as 'crystal habits'.
Based on the
cubic form and its highly symmetrical arrangement of atoms, diamond crystals
can develop into several different shapes, known as 'crystal habits'.
The most
common crystal habit is the eight-sided octahedron or diamond shape.
Diamond
crystals can also form cubes, dodecahedra, and combinations of these shapes.
Except for two shape classes, these structures are manifestations of the cubic
crystal system.
One
exception is the flat form called a macle, which is really a composite crystal,
and the other exception is the class of etched crystals, which have rounded
surfaces and may have elongated shapes.
 Real
diamond crystals don't have completely smooth faces, but may have raised or
indented triangular growths called 'trigons'.
Real
diamond crystals don't have completely smooth faces, but may have raised or
indented triangular growths called 'trigons'.
Diamonds
have perfect cleavage in four different directions, meaning a diamond will
separate neatly along these directions rather than break in a jagged manner.
The lines
of cleavage result from the diamond crystal having fewer chemical bonds along
the plane of its octahedral face than in other directions.
Diamond
cutters take advantage of lines of cleavage to facet gemstones.
Graphite is only
a few electron volts more stable than diamond, but the activation barrier for
conversion requires almost as much energy as destroying the entire lattice and
rebuilding it.
Therefore, once
the diamond is formed, it will not reconvert back to graphite because the
barrier is too high.
Under the high
pressure and temperature conditions needed to form diamond its form is actually
more stable than graphite, and so over millions of years, carbonaceous deposits
may slowly crystallize into diamond.
RELATED POSTS:
.
.
CLICK HERE .
. .
CLICK HERE . . .
 |
Multi-Media Filter, Highly-Activated Carbon Filter,
Zeolite-Process Water Softener With Brine Tank,
Fiberglass Ballast-Type Pressure Tank
(fully automatic backwash & regeneration)
|
PURICARE
Water
Treatment
Systems
.
.
...
Aganan, Pavia, Iloilo, Philippines
...
CLICK HERE . . . to view company profile . . .
http://puricare.blogspot.com/p/company-profile.html

CLICK HERE . . . to view company profile . . .
 |
| FIRSTANK Polyethelene Tanks |

 | ||
|
|















No comments:
Post a Comment